[ad_1]
Prometheus announces results for PRA023 in APOLLO-CD Phase 2 study
Prometheus Biosciences (RXDX) reported results from its ARTEMIS-UC Phase 2 and APOLLO-CD Phase 2a studies of PRA023 demonstrating strong efficacy and favorable safety results in both studies.
Based on the totality of the data in these two studies, Prometheus intends to advance PRA023 into Phase 3 studies for ulcerative colitis and Crohn’s disease in 2023.
Results from the APOLLO-CD Phase 2a Study: Prometheus’ Phase 2a APOLLO-CD clinical trial was a 12-week open-label study that enrolled 55 patients with moderate-to-severely active CD with endoscopically active disease who had failed conventional or biologic therapy.
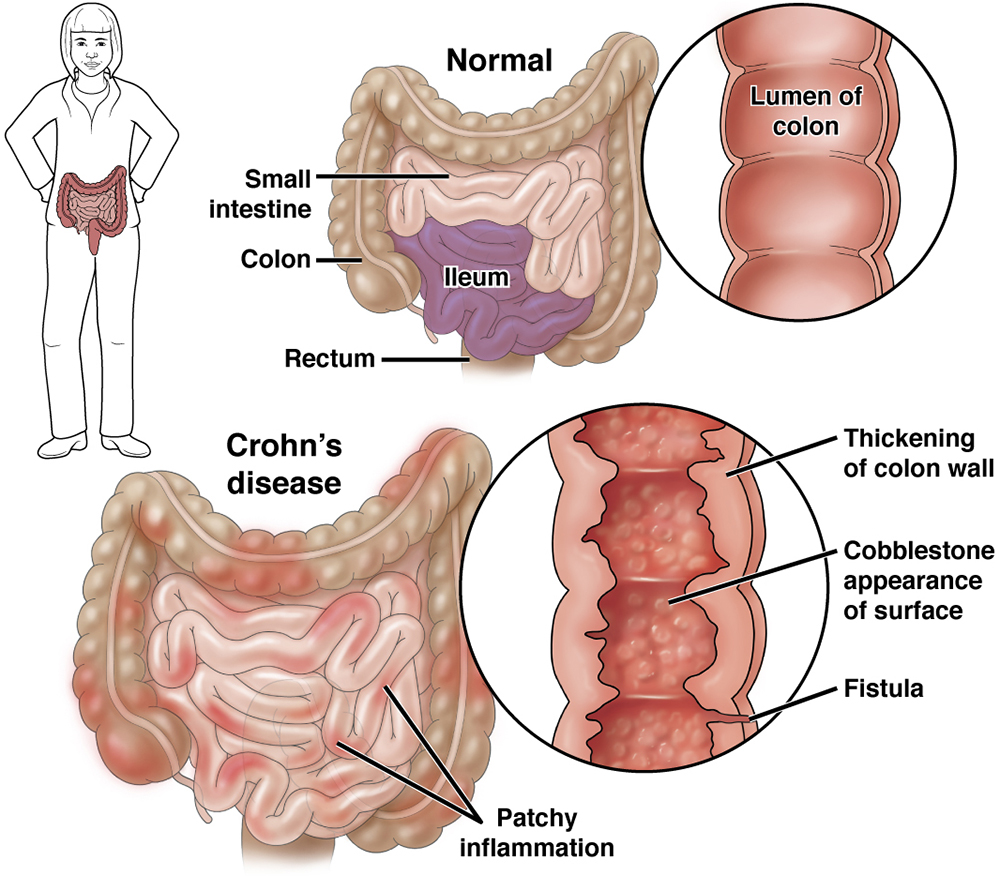
The study enrolled a highly refractory patient population with 70.9% of patients previously treated with at least one biologic therapy and 52.7% treated with two or more biologic therapies.
The results on the key endpoints were as follows: 26.0% of patients on PRA023 achieved endoscopic response; 49.1% of patients on PRA023 achieved clinical remission; PRA023 was well tolerated in the APOLLO-CD study.
There were no treatment-emergent serious adverse events, adverse events leading to discontinuation, or severe AEs assessed as related to PRA023 by the investigator.
The predictive power of the company’s prespecified genetic markers was validated using an alternative Crohn’s-specific CDx algorithm which showed 45.0% endoscopic response relative to all-comers of 26%.
While the original algorithm provided limited benefit on some of the endpoints, the alternative algorithm demonstrated enhanced performance across both clinical and endoscopic outcomes.
As a result of these positive data, Prometheus plans to advance PRA023 into pivotal development in 2023, following discussions with regulators.
Based upon confidence in its precision approach and speed to market, the company conducted an interim companion diagnostic (CDx) analysis of Cohort 1 to evaluate the effectiveness of the CDx candidate in ARTEMIS-UC. Although from limited patient numbers, data from the subset of patients who tested positive on the CDx in Cohort 1 (N=32) demonstrated a placebo-adjusted clinical remission rate of 37.5%, compared with the placebo-adjusted remission rate of 25.0% for all-comers. The expansion cohort (Cohort 2), which is statistically powered to further assess the treatment effect of PRA023 in CDx+ patients will continue to enroll, and the company expects results in the second quarter of 2023.
RXDX is up $17 to $112.86.
STOCKWINNERS
To read timely stories similar to this, along with money making trade ideas, sign up for a membership to Stockwinners.
This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility.
[ad_2]
Image and article originally from www.stockwinners.com. Read the original article here.
