[ad_1]
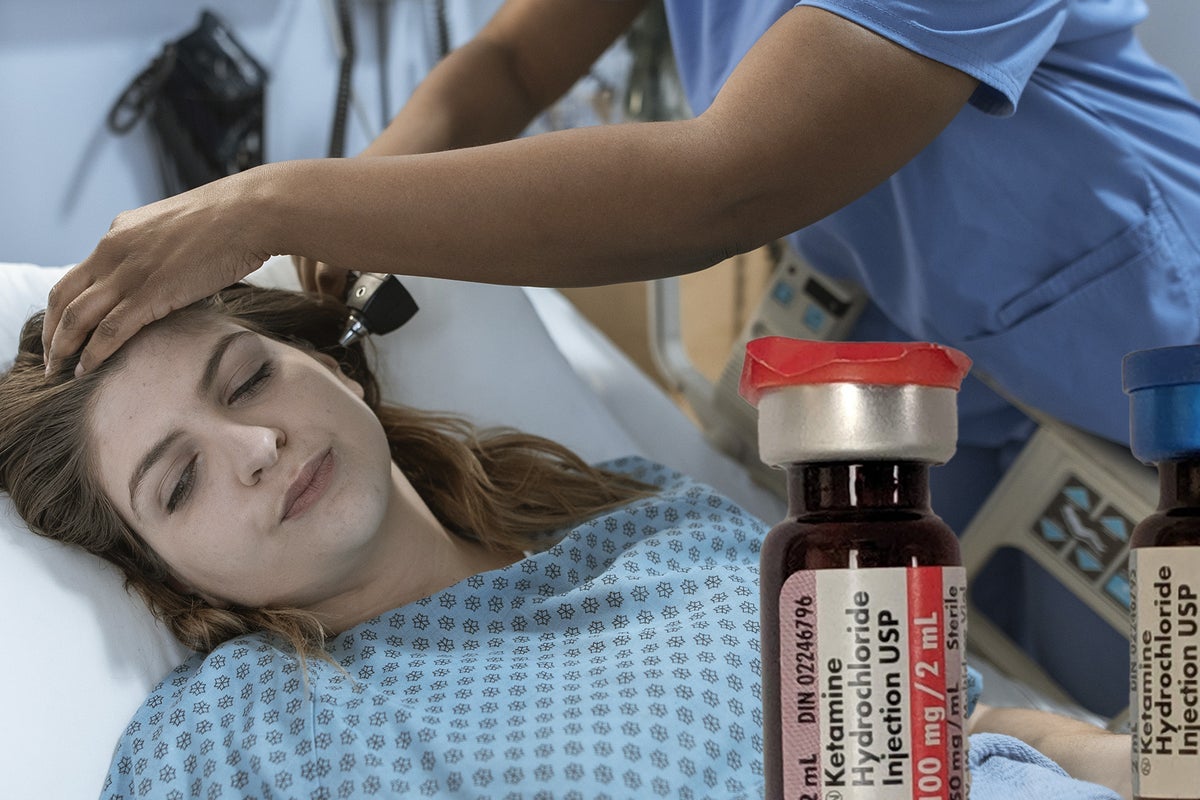
Clinical-stage biopharma psychedelics company Seelos Therapeutics Inc. SEEL announced it has dosed the first patients in a Phase 1 study with healthy adult Japanese and non-Asian participants to compare the safety and pharmacokinetic (PK) profiles of proprietary intranasal racemic ketamine SLS-002.
The company has previously consulted and received an endorsement to conduct the ethno-bridging trial on behalf of Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) as well as the FDA.
Seelos chairman and CEO Dr. Raj Mehra says the study is “an important first step” in evaluating the novel drug’s potential in patients globally. “Our market research suggests a high unmet global need for a therapy with both antidepressant and anti-suicidal effects.”
Specifically, the trial will assess dosage and administration, sample size, inclusion and exclusion criteria, endpoints and blood sampling, with data expected to help inform the inclusion of Japanese subjects in the design of a global trial in patients with Major Depressive Disorder (MDD) at imminent risk of suicide.
SLS-002 holds two Investigational New Drug (IND) applications for the treatment of acute suicidal ideation and behavior in MDD and in PTSD.
The compound was originally derived from a Javelin Pharmaceuticals Inc./Hospira Inc. program with 16 clinical studies involving approximately 500 subjects. Its current clinical development program includes two parallel Phase 1 studies which will be followed by pivotal registration studies after meeting with the FDA.
Seelos is looking to provide a therapy with SLS-002 for the treatment of suicidality in the US, considering there were over one million visits to emergency rooms for suicide attempts in the country in a year (2019).
Photo: Benzinga edit with photo by RODNAE Productions on Pexels and Doc James on Wikimedia Commons.
Join us at our second edition of the Benzinga Psychedelics Capital Conference at the Fontainebleau Miami Beach Hotel in Florida on April 13.
This is THE place to get DEALS DONE, raise money, jumpstart M&A and meet investors and key partners.
Secure your tickets now. Prices are going up very soon.
[ad_2]
Image and article originally from www.benzinga.com. Read the original article here.