[ad_1]
Drug discovery company Mindset Pharma MSSTF has been greenlighted by the US Patent and Trademark Office (USPTO) on its application “Psilocin Derivatives As Serotonergic Psychedelic Agents for the Treatment of CNS Disorders.”
The application includes the company’s psilocybin analogs lead drug candidate MSP-1014, its backup MSP-1009 and additional pro-drug candidates belonging to the family 1 tryptamine drug compounds.
Both MSP-1014 and MSP-1009 demonstrated improved efficacy and safety profiles with reduced potential side effects and evidence of increased target engagement in preclinical studies.
“Our early and consistent focus on novelty has created a valuable intellectual property (IP) portfolio. This focus on IP has already created value for Mindset, and today’s announcement is a good indication of the potential for additional returns for Mindset thanks to our ongoing focus on novel optimized psychedelic-inspired medications,” concluded CEO James Lanthier.
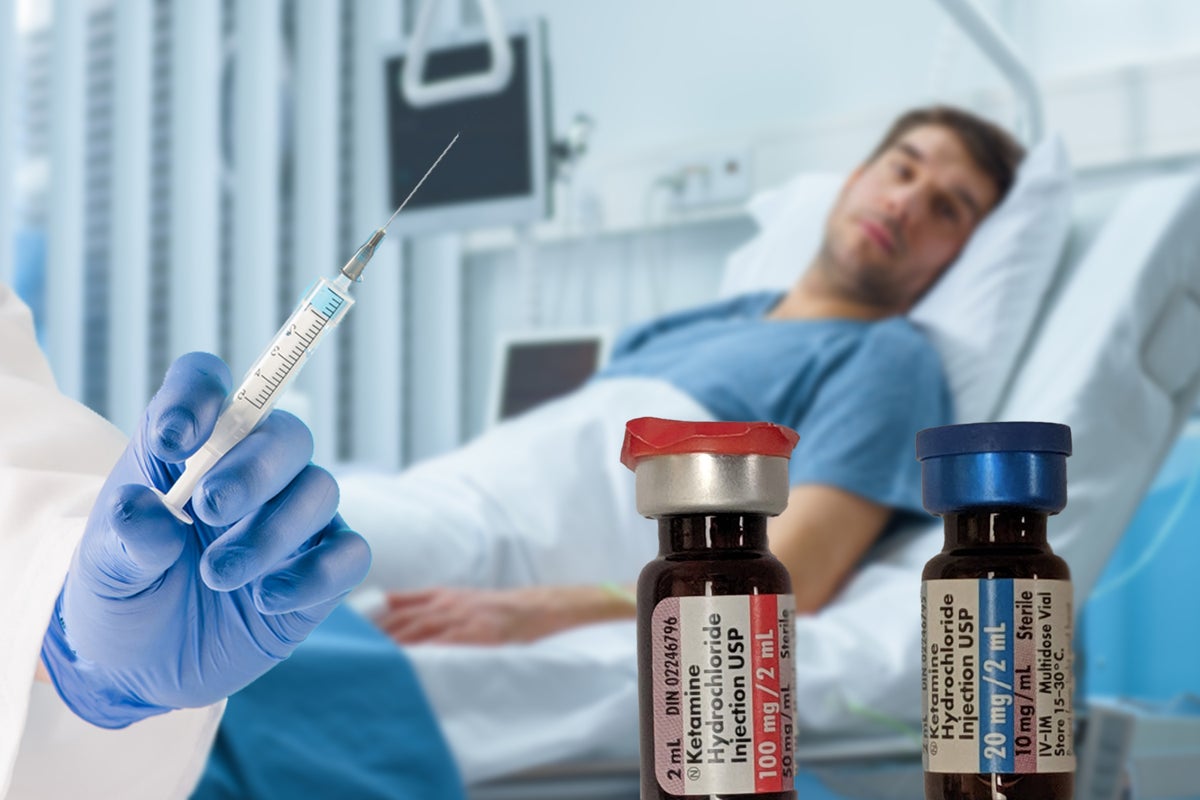
PharmaTher Holdings PHRRF has applied with the FDA to receive Orphan Drug Designation (ODD) on ketamine therapy for Rett Syndrome.
This ODD would qualify ketamine for certain benefits and incentives, including seven years of marketing exclusivity for the targeted indication, potential tax credits for certain clinical drug costs, eligibility for orphan drug grants, and the waiver of the FDA New Drug Application filing fee (approx. $2.4 million.)
See also: Pharmather Lands US Patent To Formulate, Produce Ketamine With This New Process
A recent Phase 2 trial assessed ketamine treatment for this rare genetic neurological disorder. Now, the company will evaluate the study’s unpublished results in support of a potential Phase 3 trial and an FDA-agreed regulatory plan.
PharmaTher currently holds four orphan drug designations granted by the FDA for proprietary racemic ketamine KETARX, including ischemia-reperfusion injury from organ transplantation and Status Epilepticus.
Biopharma home-based solutions company Bexson Biomedical Inc. announced that the USPTO issued patent “Complexing Agent Salt Formulations of Pharmaceutical Compounds” corresponding to its proprietary formulation technology SEVALENT.
The novel technology is the basis for Bexson’s lead therapy BB106 on ketamine for postoperative pain management, a leading driver of opioid addiction.
Now, the new patent expands its potential utility to additional small molecules, enabling intravenous-only therapies to be delivered subcutaneously in a controlled manner for at-home use.
Photo: Benzinga edit with photo by True Touch Lifestyle and Gorodenkoff on Shutterstock and Doc James on Wikimedia Commons.
[ad_2]
Image and article originally from www.benzinga.com. Read the original article here.